Natasha Kekre1, Kevin A. Hay2, John Webb3, Miruna Bala2, Mhairi Sigrist2, Anne-Marie Clement1, Manoj M. Lalu1, Dean A. Fergusson1, John C. Bell1, Harold Atkins1, Brad H. Nelson3, Robert A. Holt2
1 Ottawa Hospital Research Institute, Ottawa, ON
2 BC Cancer, Vancouver, BC
3 BC Cancer, Victoria, BC
Background: CAR-T cell therapy has proven effective in treating adult patients with relapsed/refractory CD19 positive B cell malignancies that have failed standard therapies, but access to commercial CD19 CAR-T remains limited in Canada.
Purpose: We designed a multicenter, two-stage, single-arm, open-label early phase study to determine the safety and efficacy of Canadian-made CD19 CAR-T cells (CLIC-1901) in participants with CD19+ ALL, CLL and NHL.
Methodology: The anti-CD19 CAR transgene, developed in Vancouver (BC Cancer), contained a 4-1BB costimulatory domain. Lentivirus containing the transgene was produced at the Ottawa Biotherapeutics Manufacturing Center. Peripheral blood mononuclear cells were collected by leukapheresis in Ottawa and Vancouver and shipped to the CAR-T cell manufacturing facility in Victoria. T cells were selected, activated, transduced, and expanded using a GMP compliant semi-automated, closed process using the Miltenyi Prodigy. Final CLIC-1901 product was tested for identity, potency, purity, and sterility, and only infused if release criteria met. Participants underwent lymphodepletion with fludarabine (40 mg/m2/d x 3) and cyclophosphamide (500 mg/m2/d x 2), prior to infusion of >1e6CAR expressing cells per kilogram of body weight (maximum 2e8 total CAR expressing cells) non-cryopreserved CLIC-1901.
Results: Of 48 patients screened for eligibility, 35 were enrolled. 5 enrolled participants did not receive CLIC-1901 due to manufacturing failures early after protocol launch (n=2, resolved with first protocol amendment), severe myocarditis before lymphodepletion (n=1), and death before infusion (n=2). 30 participants received CLIC-1901 CAR-T therapy: 21 males (70%), median age 66 (range 18-75). The median number of prior therapies was 3 (range 2-6). 13 (43%) patients had failed a stem cell transplant (allogeneic (n=5), autologous (n=6), both (n=2)). The disease indication was DLBCL (n=10), MCL (n=8), transformed DLBCL (n=4), ALL (n=5), follicular lymphoma (n=1), Richter’s transformation (n=1) and plasmablastic lymphoma (n=1). The time from enrollment to CLIC-1901 infusion was a median of 20 days (range 15-48). The median CLIC-1901 dose infused was 2.3e6 CAR-T cells/kg (range 1.3e5-3.6e6/kg). Toxicity included CRS (grade 1 n=9, grade 2 n=7, grade 3 n=1, and grade 5 n=1), at median onset of 1.5 days after CLIC-1901 infusion (range 0-9 days). ICANS occurred in 2 participants (grade 4 n=1, and grade 5 n=1). At a median follow-up of 4 months (IQR 4-7), the median progression-free survival was 5 months (95% CI 4-not estimable). Figure 1 shows patient level data.
Conclusion: This is the first trial of Canadian-made CAR-T cells and demonstrates that Canadian manufacturing of vector, virus, and T cells is feasible within the academic sphere in Canada. Our preliminary results indicate that CLIC-1901 is safe and tolerable. Longer follow-up and more patients are needed to determine overall efficacy.
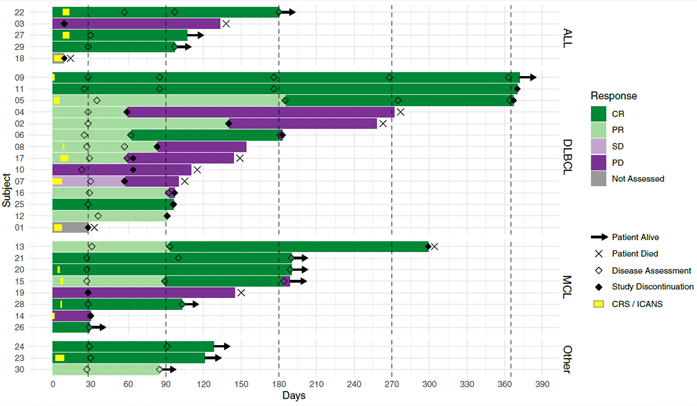 Swimmer’s plot of first 30 patients treated with CLIC-1901 from time of cell infusion
Swimmer’s plot of first 30 patients treated with CLIC-1901 from time of cell infusion
